Chugaev elimination
| Chugaev elimination | |
|---|---|
| Origin of the name | Lev Chugaev |
| Kind | Elimination reaction |
| Identification information | |
| RSC ontology ID | RXNO: 0000538 |
Chugaev elimination (Chugaev だつり, Chugaev elimination) is a reaction to pyrolize O-alkyl xanthate with kind of the elimination reaction in the organic chemistry, and to get alkene. Or it is a dehydration reaction to get alkene from first class alcohol as a key by the reaction (lower expression). This name is associated with Russian chemist, lev アレクサンドロヴィチ Chugaev (Lev Aleksandrovich Chugaev, from 1873 to 1922) [1].
At first O-alkyl xanthic acid anion is enacted when I trigger carbon disulfide to alkoxide which I produced with a base from alcohol. I let iodomethane coexist then and assume it S-methyl ester.
A concerted syn elimination happens like a lower expression, and alkene is provided when I heat this ester to around 200 degrees Celsius. Through a transition state of the 6-membered ring form, hydrogen atoms on β-carbon transpose 1,5-to the sulfur top. The ester to by-produce with alkene disintegrates successively to sulfuration carbonyl (O=C=S) and methanethiol (CH3SH).
References
- Latscha, H. P., Chemie-Basiswissen., Berlin(2002): Springer.
- ^ Chugaev, L. Ber. Dtsch. Chem. Ges. 1899, 32, 3332.
This article is taken from the Japanese Wikipedia Chugaev elimination
This article is distributed by cc-by-sa or GFDL license in accordance with the provisions of Wikipedia.
In addition, Tranpedia is simply not responsible for any show is only by translating the writings of foreign licenses that are compatible with CC-BY-SA license information.


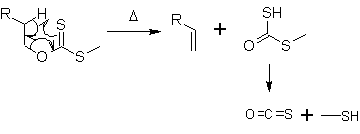

0 개의 댓글:
댓글 쓰기