Cyanamid
| Cyanamid | |
|---|---|
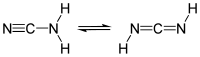 | |
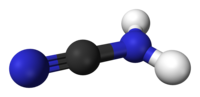 | |
| Cyanamide, | |
| Another name Amidocyanogen, carbamonitrile, carbimide, carbodiimide, cyanoamine, cyanoazane, N-cyanoamine, cyanogenamide, cyanogen nitride, hydrogen cyanamide | |
| Identification information | |
| CAS registration number | 420-04-2 |
| PubChem | 9864 |
| ChemSpider | 9480 |
| UNII | 21CP7826LC |
| EINECS | 206-992-3 |
| United Nations / North America number | |
| KEGG | D00123 |
| ChEMBL | CHEMBL56279 |
| RTECS number | GS5950000 |
| |
| | |
| Characteristic | |
| Chemical formula | CH2N2 |
| Molar mass | 42.040 g/mol |
| The appearance | Colorless acicle |
| Density | 1.28 g/cm3 |
| Melting point | 44 degrees Celsius, 317 K, 111 degrees Fahrenheit |
| The boiling point | 260 degrees Celsius, 533 K, 500 degrees Fahrenheit (the resolution |
| Solubility to water | 77.5g/100ml (15 degrees Celsius) 85g/100ml (25 degrees Celsius) |
| Solubility to an organic solvent | Soluble |
| The risk | |
| Safe data sheet (outside link) | ICSC 0424 |
| EU classification | Toxic (T) |
| EU Index | 615-013-00-2 |
| NFPA 704 | |
| R phrase | R21 R25 R36/38 R43 |
| S phrase | S1/2 S3 S22 S36/37 S45 |
| Ignition point | 141 degrees Celsius, 414K |
| Associated material | |
| Related material | Calcium cyanacium |
| I can put a case, the data without the special mention for normal temperature (25 degrees Celsius), the ordinary pressure (100 kPa). | |
Cyanamid (Cyanamide) is an amide compound expressed in molecular formula CN2H2. It is a material used as formation product raw materials and manure and is used as pharmaceutical products.
The factory which produced approximately 20,000 tons a year in 1910 caused Germany Italy Canada France and was operated in Japan. Amount of production increased to 10 times in 1913, and the yearly output rose to 600,000 tons for manure under the wartime and nitrogen demand for military demand in 1918. Amount of production increased as materials of the melamine resin from 1939. In 1962, the world total amount of production exceeded 9 million tons. The main country of origin of this time was Germany, Japan, the United States.
Table of contents
Manufacturing method
I react calcium cyanacium with water, and a cyanamid water solution is provided when I turn on carbon dioxide in solution. I am neutralized with sulfuric acid and precipitate calcium carbonate and am provided as colorless acicle when I concentrate it.
- CaCN2 + H2O + CO2 → CaCO3 + CN2H2
Property
The cyanamid has carbodiimide and tautomerism. The dicyandiamide which cyanamid and carbodiimide reacted to is formed when I heat up in alkaline solutions. The dicyandiamide gradually disintegrates with water in the soil and becomes urea and the ammonium carbonate. Because dicyandiamide oneself is harmful to a plant, it is used as base manure.
Because the cyanamid becomes guanidine in response to amine, it is important as formation product raw materials.
Cyanamid oneself causes inflammation with acridity when I touch the skin. I am appointed to dynamite in Deleterious Substance Control Law in Japan.
Pharmaceutical products
The cyanamid is used in abstinence from alcoholic drinks, a temperance in drinking purpose for chronic alcoholism, a hyperdrinker with a Japanese Pharmacopoeia publication product. The brand name is Shea live id.
Action mechanism
Because this agent inhibits aldehyde dehydrogenase, I control the ethanol metabolism in the liver and let acetaldehyde causing the hangover accumulate in the body. Therefore, even a little liquor has a painful experience, and abstinence from alcoholic drinks, an effect of the temperance in drinking are brought.
Instructions
I took in food containing ethanol such as pickles seasoned with sake lees and a little alcoholic drinks with medical properties and may feel sick from drinking it even if I do not drink the liquor which is luxury goods at the time of the remedy with this agent. Attention is necessary for an alcohol intake of the degree not to say until "drinking".
Allied item
- Manure, a pesticide. I am in danger when I drink after fertilization (dispersion) because I include cyanamid. After fertilization (dispersion), you should avoid drinking for around 24 hours
Outside link
This article is taken from the Japanese Wikipedia Cyanamid
This article is distributed by cc-by-sa or GFDL license in accordance with the provisions of Wikipedia.
In addition, Tranpedia is simply not responsible for any show is only by translating the writings of foreign licenses that are compatible with CC-BY-SA license information.

0 개의 댓글:
댓글 쓰기