Aquaporin
The aquaporin (Aquaporin, AQP) is the protein with the pore (pore) which there is to a cell membrane. It is kind of the main membrane protein belonging to the MIP (major intrinsic proteins) family [1].
Because I can pass only a water molecule selectively, I am related to uptake of the water to a cell.
Some exist a disease to be caused by the abnormality of the aquaporin gene [2]; [3]. Peter a went wrong, and (Peter Agre) won Nobel Prize in Chemistry of 2003 by discovery of the aquaporin [4]. I won Roderick McKinnon (Roderick MacKinnon) by structure of the potassium channel and a study of the mechanism jointly then [5].
Table of contents
Function
The aquaporin permeabilizes a water molecule selectively, but an ion and other materials are called aquaporin (water channel) not to permeabilize [6].
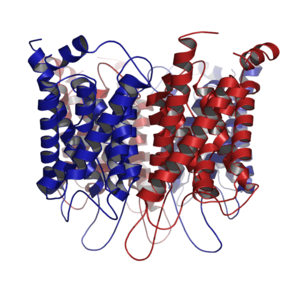
The aquaporin is comprised of the same subunit of four normal, and each monomer acts as aquaporin. The water molecule passes the pore of this channel. Because this aquaporin acts, cell membrane permeability of the water only costs.
Human many cells, certain bacteria, a system transporting such a water molecule is more indispensable for the organic living entity such as the plant [7].
Discovery
With most cells, the water goes in and out of a cell by passing the lipid of the cell membrane. However, it was expected what water went in and out of by some different mechanism because permeability of the water was high with some epithelium cells. I stopped by to go wrong and was discovered "aquaporin 1" (aquaporin-1) a that (called CHIP at first) was in the Johns Hopkins University in those days of the aquaporin first afterwards. The discovery of this first aquaporin was 1992 [8].
A went wrong, and the と coworker was conferred Nobel Prize in Chemistry of 2003 on by this epoch-making discovery and study of the aquaporin [5]. I succeeded in analyzing three-dimensional structure of aquaporin 1 in detail in cooperation with other study teams in 1999 [9]. Furthermore, it parsed how you let only a water molecule go through in a pore by the simulation using the super computer [10].
Structure
Aquaporin
The aquaporin includes the alpha helix structure of six right-hand direction-facing objects, and N-terminus and the C-terminus push it out on the cell membrane surface on the cytoplasm side [6]; [11]. Half sequence of an N-terminal side and the C-terminal side is similar, and sequence exists repeatedly. There is the researcher who thinks that this stollen is the thing that a gene of half size repeated for an initial stage of the evolution.
Five loop structure (A-E) exists between ヘリックス and do the cell outside and intracellular communication. The loop B and E is hydrophobic and includes the structure called the Asn-Pro-Ala (NPA) motif.
The NPA motif is piled up in the lipid double layer inside and does three-dimensional funnel-formed structure. This part passes a water molecule. One is called a channel clamping site (channel constriction site) of the peptide among these two parts piled up. The other does narrower form and is called ar/R choice filter (ar/R selectivity filter).
The aquaporin is comprised of a tetramer in a cell membrane and passes water (the small material which is not tinged with an electric charge and sometimes: glycerol, carbon dioxide, ammonia, urea). Peptide sequences are different in the different aquaporin of the kind, and the size of the pore is thereby different each, too. The numerator that I can pass by the size of the pore is limited and puts only the small molecules such as the water molecule in the small pore. However, I can keep the electrochemical potential of the film because I was charged with an electric charge, and (electrons) does not let it go through.
NPA motif
It was revealed that the water molecule passed a channel to one line by the simulation using the computer. The water molecule passes a small channel by adapting to the electric field formed by the atom of the wall of the channel.
When the water molecule is in the channel, I turn an oxygen atom to the lower direction. Orientation is reversed with the intermediate part, and an oxygen atom turns to the top. The rotary motion of the water in this pore depends on the hydrogen bonding between the oxygen atom of asparagine and the water molecule of two NPA motifs. The water molecule is in the channel with one line downward and appears upward. I hold the water molecule transit rate of the channel by keeping away a water molecule for reverse by Grotthuss mechanism (Grotthuss mechanism) [12].
ar/R choice filter
The ar/R (aromatic / arginine: aromatic/arginine) choice filter removes the molecules which are going to be in the pore with binding to the water molecule. By this work, molecules except the water cannot pass with a channel. There is the ar/R choice filter on both sides of the NPA motif and is comprised of ヘリックス 2 (H2) and 5 (H5) two amino acid residue and two residues of loop E (LE1, LE2). There is it at the entrance of the cell outside and is usually on 8Å of the NPA motif. In addition, I form the thinnest part of the pore. This structure has a function of to weaken hydrogen bonding water, but does not influence interaction with water and arginine (the proton filter plays its part) of the positive charge.
Mammalian aquaporin
As for the aquaporin which mammals have, 13 kinds are known; six kinds of those in the kidney [13]. However, the possibility that more kinds exist is doubted.
The best known aquaporin is a kind below.
The water passes a cell membrane by passing a lipid double layer and aquaporin. Most of the aquaporin do not let a material except the water go through. Some aquaporin is called アクアグリセロポリン (aquaglyceroporin) and maintain water, glycerol, some other small numerators.
Comparison
| Kind | Position [14] | Function [14] |
|---|---|---|
| Aquaporin 1 |
| It is reabsorbed the water |
| Aquaporin 2 |
| I reabsorb water depending on バソプレシン |
| Aquaporin 3 |
| It is reabsorbed the water |
| Aquaporin 4 |
| It is reabsorbed the water |
Aquaporin of the plant
The plant carries the water which I drew up from a root to the whole through a vascular strand. These two courses are known to exist. It is apoplastic (Apoplast) and a symplastic (Symplast) course. The aquaporin is thought to promote transportation of the water by the symplast. When a plant is exposed to a mercurous chloride (inhibitor of the aquaporin), the transport volume of the water decreases, but the transport volume of the ion does not decrease. Therefore, it is thought that transportation of the water and the transportation of the ion become independent each.
With four kinds as for the aquaporin of the plant below [15].
- PIP (Plasma membrane Intrinsic Protein)
- TIP (Tonoplast Intrinsic Protein) [16]
- NIP (Nodulin-26 like Intrinsic Protein) [17]
- SIP (Small basic Intrinsic Protein) [18]
Gating of the aquaporin
The gating (The gating of aquaporins) of the aquaporin is carried out to stop a flow of the water of the pore. It is thought that this is performed at time when quantity of the water in a cell decreased by droughts.
It is thought that the gating of the aquaporin is carried out by preventing gate mechanism and a protein tertiary structure of the aquaporin changing and from letting water go through. At least two kinds of gating is carried out with the root.
These are expected by the protonation to the dephosphorylation of the specific serine residue and a specific histidine residue when it takes place. The phosphorylation of the aquaporin is related to flowering and the shut flower of the plant.
PIP
Protein peculiar to a plasma-membrane is discovered and is called PIP (Plasma membrane Intrinsic Protein). PIP is divided into PIP1 and PIP2 in the difference in peptide sequences. PIP1 has a lower transportation capability of the water than PIP2, but the transport volume of the water increases when PIP1 becomes PIP2 and the tetramer.
Aquaporin and disease
Two cases attributable to the variation of the aquaporin were found obviously.
- By aquaporin 2 genetic mutation, hereditary Diabetes inositus happened.
- The mouse which let an aquaporin 0 gene mutate suffered from a congenital cataract.
It is thought that Homo sapiens of a pole has little aquaporin 1. They are usually healthy, but ability to reabsorb water when the fluid volume of the body decreased is low. The mouse which knocked out aquaporin 1 showed a similar symptom.
I am called optic nerve myelitis or Devic's disease, and the autoimmune disease for aquaporin 4 repeats acute optic nerve flame and acute myelitis. It was thought with a subtype of the multiple sclerosis, but in late years is established as an independent disease concept.
Reference
- ^ Agre P (2006). "The aquaporin water channels." Proc Am Thorac Soc 3 (1): 5–13. doi: 10.1513/pats.200510-109JH. PMID 16493146.
- ^ Agre P, Kozono D (2003). "Aquaporin water channels: molecular mechanisms for human diseases." FEBS Lett. 555 (1): 72–8. doi: 10.1016/S0014-5793(03) 01083-4. PMID 14630322.
- ^ Schrier RW (2007). "Aquaporin-related disorders of water homeostasis." Drug News Perspect. 20 (7): 447–53. doi: 10.1358/dnp.2007.20.7.1138161. PMID 17992267.
- ^ Knepper MA, Nielsen S (2004). "Peter Agre, 2003 Nobel Prize winner in chemistry." J. Am. Soc. Nephrol. 15 (4): 1093–5. doi: 10.1097/01.ASN.0000118814.47663.7D. PMID 15034115.
- ^ a b "The Nobel Prize in Chemistry 2003". Nobel Foundation. January 23, 2008 reading.
- ^ a b Gonen T, Walz T (2006). "The structure of aquaporins." Q. Rev. Biophys. 39 (4): 361–96. doi: 10.1017/S0033583506004458. PMID 17156589.
- ^ Kruse E, Uehlein N, Kaldenhoff R (2006). "The aquaporins." Genome Biol. 7 (2): 206. doi: 10.1186/gb-2006-7-2-206. PMID 16522221.
- ^ Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S (1993). "Aquaporin CHIP: the archetypal molecular water channel". Am. J. Physiol. 265 (4 Pt 2): F463–76. It is . PMID 7694481
- ^ Mitsuoka K, Murata K, Walz T, Hirai T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (1999). "The structure of aquaporin-1 at 4.5-A resolution reveals short alpha-helices in the center of the monomer." J. Struct. Biol. 128 (1): 34–43. doi: 10.1006/jsbi.1999.4177. PMID 10600556.
- ^ de Groot BL, Grubmüller H (2005). "The dynamics and energetics of water permeation and proton exclusion in aquaporins." Curr. Opin. Struct. Biol. 15 (2): 176–83. doi: 10.1016/j.sbi.2005.02.003. PMID 15837176.
- ^ Fu D, Lu M (2007). "The structural basis of water permeation and proton exclusion in aquaporins." Mol. Membr. Biol. 24 (5-6): 366–74. doi: 10.1080/09687680701446965. PMID 17710641.
- ^ Tajkhorshid E, Nollert P, Jensen MØ, Miercke LJ, O'Connell J, Stroud RM, Schulten K (2002). "Control of the selectivity of the aquaporin water channel family by global orientational tuning." Science 296 (5567): 525–30. doi: 10.1126/science.1067778. PMID 11964478.
- ^ Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA (2002). "Aquaporins in the kidney: from molecules to medicine." Physiol. Rev. 82 (1): 205–44. doi: 10.1152/physrev.00024.2001. PMID 11773613.
- ^ a b Unless else specified in table boxes, then ref is: Walter F., PhD. Boron. Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 1-4160-2328-3. Page 842
- ^ Kaldenhoff R, Bertl A, Otto B, Moshelion M, Uehlein N (2007). "Characterization of plant aquaporins." Meth. Enzymol. 428: 505–31. doi: 10.1016/S0076-6879(07) 28,028-0. PMID 17875436.
- ^ Maeshima M (2001). "TONOPLAST TRANSPORTERS: Organization and Function." It is 469–497. doi: Annu Rev Plant Physiol Plant Mol Biol 52 10.1146/annurev.arplant.52.1.469. PMID 11337406.
- ^ Wallace IS, Choi WG, Roberts DM (2006). "The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins." Biochim. Biophys. Acta 1758 (8): 1165–75. doi: 10.1016/j.bbamem.2006.03.024. PMID 16716251.
- ^ Johanson U, Gustavsson S (2002). "A new subfamily of major intrinsic proteins in plants". Mol. Biol. Evol. 19 (4): 456–61. It is . PMID 11919287
Allied item
Outside link
- Animation(MPEGFile)
- Bowen R. "Aquaporins: Water Channels". Colorado State University. January 23, 2008 reading.
- Mallery C. "Aquaporins - Water Channels". University of Miami. January 23, 2008 reading.
- Harrison N. "Describe how aquaporins enable water to cross cell membranes. Comment on the physiological roles of AQP and of related transporters". January 23, 2008 reading.
- Computational Biomolecular Dynamics Group. "Aquaporin movies and pictures". Max Planck lnstitute. January 23, 2008 reading.
- Orientation of Proteins in Membranes Group. "Aquaporin movies and pictures: Calculated positions of aquaporins of known 3D structure in membrane". University of Michigan. January 23, 2008 reading.
- Theoretical and Computational Biophysics Group. "Structure, Dynamics, and Function of Aquaporins". University of Illinois at Urbana-Champaign. January 23, 2008 reading.
- Protein structure data bank Molecules 173 of this month aquaporin (Aquaporin):
- Aquaporin - brain science dictionary
This article is taken from the Japanese Wikipedia Aquaporin
This article is distributed by cc-by-sa or GFDL license in accordance with the provisions of Wikipedia.
In addition, Tranpedia is simply not responsible for any show is only by translating the writings of foreign licenses that are compatible with CC-BY-SA license information.



0 개의 댓글:
댓글 쓰기