セリチニブ
 | |
| Material name by the IUPAC glossology | |
|---|---|
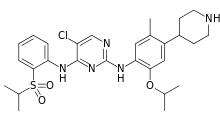
| 5-Chloro-N2-[2-isopropoxy-5-methyl-4-(4-piperidinyl) phenyl] -N4-[2-(isopropylsulfonyl) phenyl] -2,4-pyrimidinediamine | |
| Clinical data | |
| Brand name | Zykadia |
| AHFS/Drugs.com | Multum Consumer Information |
| Fetus degree of risk classification |
|
| Legal regulation |
|
| Dosage method | Oral |
| Identification | |
| CAS number | 1,032,900-25-6 |
| ATC cord | L01XE28 |
| PubChem | CID: 57379345 |
| DrugBank | DB09063 |
| ChemSpider | 29315053 |
| KEGG | D10551 |
| ChEBI | CHEBI: 78432 |
| ChEMBL | CHEMBL2403108 |
| Another name | LDK378 |
| Chemical data | |
| Chemical formula | C28H36ClN5O3S |
| Molecular weight | 558.14 g/mol |
| |
| |
セリチニブ (Ceritinib, a brand name: ジカディア, a development cord: LDK378) is one of the therapeutic drugs for some lung cancer [1]. Undifferentiated lymphoma kinase () has inhibition action (ALK) [2]. It was approved as a therapeutic drug of the ALK-positive non-small-cell lung cancer with the metastasis after the クリゾチニブ treatment in the United States in April, 2014 by the U.S. Food and Drug Administration (FDA) [1]. "The non-small-cell lung cancer of the unresectable progress, recurrence resistant to クリゾチニブ or with positive ALK fused gene of the non-tolerance" was approved as adaptation in Japan in March, 2016 [3].
Side effect
The serious side effect listed in an attached document is extension (5.7%), bradycardia (0.7%), severe diarrhea (6.4%), hyperglycosemia (0.7%), diabetes (0.7%), pancreatitis for a stroma-related lung disease (1.4%), a liver function disorder (3.6%), a QT interval (frequency ignorance); [4]. Because these side effects are sometimes fatal, a notice matter (proper use information) for the purpose of the ensuring safety of the patient is notified medical personnel of on the approval same day by Ministry of Health, Labour and Welfare [5].
An appetite decline, nausea (77.9%), diarrhea (77.1%), vomiting (58.6%), abdominal pain, liver function test level abnormality (ALT (GPT) increase, AST (GOT) increase, blood bilirubin increase), fatigue are accepted elsewhere by more than 20% of patients.
Action mechanism
The ALK is a kind of the tyrosine kinase controlling the increase of the cell. I have dormancy, but the fusion of an ALK gene and other genes is seen in 3-5% of the non-small-cell lung cancer, and cell proliferation signals always become ON because an ALK gene emerges constantly, and abnormality increases usually produce it [6]. Such a cancer is called ALK-positive (ALK+) non-small-cell lung cancer. The ALK repressor inhibits this kinase and stops an increase and induces apoptosis.
References
- ^ a b "FDA Approves Ceritinib for ALK-Positive Lung Cancer." Medscape. (April 29, 2014)
- ^ Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, Camidge DR, Vansteenkiste J, Sharma S, De Pas T, Riely GJ, Solomon BJ, Wolf J, Thomas M, Schuler M, Liu G, Santoro A, Lau YY, Goldwasser M, Boral AL, Engelman JA (2014). "Ceritinib in ALK-Rearranged Non–Small-Cell Lung Cancer." It is doi: 1189-1197 N Engl J Med 370 10.1056/NEJMoa1311107. PMID 24670165.
- ^ "ALK repressor "ジカディア ®," production sale approval in Japan is acquired as a therapeutic drug of the ALK fused gene-positive non-small cell lung cancer". Novartis (March 28, 2016). May 20, 2016 reading.
- ^ "ジカディアカプセル 150 mg attached document"(March, 2016). May 20, 2016 reading.
- ^ Ministry of Health, Labour and Welfare (March 28, 2016). "A notice matter on using the セリチニブ preparation" (PDF). Japanese clinical tumor Pharmaceutical Society. May 20, 2016 reading.
- ^ "discovers a therapeutic drug-resistant cause for the ALK-positive lung cancer" (PDF). A public interest cancer meeting for the study (December 25, 2015) Foundation. May 20, 2016 reading.
This article is taken from the Japanese Wikipedia セリチニブ
This article is distributed by cc-by-sa or GFDL license in accordance with the provisions of Wikipedia.
In addition, Tranpedia is simply not responsible for any show is only by translating the writings of foreign licenses that are compatible with CC-BY-SA license information.
0 개의 댓글:
댓글 쓰기