1,1,1-propellane
| [1.1.1]Propellane | |
|---|---|
 | 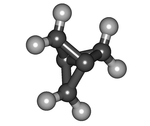 |
| Tricyclo[1.1.1.01,3]pentane | |
| Identification information | |
| CAS registration number | 35,634-10-7 |
| ChemSpider | 125285 |
| |
| | |
| Characteristic | |
| Chemical formula | C5H6 |
| Molar mass | 66.1 g mol-1 |
| I can put a case, the data without the special mention for normal temperature (25 degrees Celsius), the ordinary pressure (100 kPa). | |
[1.1.1]Propellane (1.1.1-Propellane) is an organic compound and is simplest propellane. It is a hydrocarbon of chemical formula C5H6 or C2(=CH2)3. I have molecular structure sharing C-C combination having one three three-membered rings.
[1.1.1]Propellane is the molecule which a distortion has a very big. The bond of two central carbon atoms is reverse all sides figure, and the length of the central combination is 160 pm. Strength of the combination is controversial, and there is the strength until the opinion which there is not at all from 59-65 kcal/mol. If 80 kcal/mol is high, the energy of the biradical state is calculated. When this compound becomes 114 degrees Celsius with instability very much, I isomerize it to 3-methylene cyclo butene voluntarily in the half-life of five minutes. The distortion energy is estimated to be 102 kcal/mol.
Table of contents
Synthetic
[1.1.1]Propellane was composed by the following schemes for the first time in 1982 by Kenneth Y Bergh and Frederick Walker [1].
At first 1,3-dicarboxylic acid 1 of bicyclo[1.1.1]pentane is converted into dibromide 2 equivalent by Hunsdiecker reaction, and coupling reactions with N-butylithium occur next. End-product 3 is isolated at -30 degrees Celsius by column chromatography.
However, the much simpler synthetic method is announced [2]. In that way, 7 [3] that at first the bromocarbene adds it to an alkene bond of 3-chloro-2-(chloromethyl) propene 6, and I am deprotonated by methyl lithium, and nucleophilic substitution produces afterwards. I stay in solution of -196 degrees Celsius without being isolated.
Reaction
Acetic acid addition
[1.1.1]Propellane produces methylene cyclobutane ester in response to acetic acid voluntarily (4 of the above).
Polymerization
Propellane polymerizes it by central C-C combination dissociating, and being combined with the next monomer units [1.1.1] and forms so-called studio fan [4].
Radical polymerization begins by methyl formate and benzoyl peroxide, and various oligomers are formed. By the anion polymerization with N-butylithium, a complete polymerization product is provided. It was shown that the C-C-binding bond head connected by X-ray diffraction of the polymer was 148 only pm.
I polymerize 1,3-dehydroadamantane which I can consider to be cross-linked [1.3.3] propellane equally.
Allied item
Source
- ^ K. B. Wiberg, F H. Walker (1982), [1.1.1]Propellane. J. Am. Chem. Soc. volume 104 issue 19, pp. 5239-5240; doi: 10.1021/ja00383a046
- ^ J. Belzner, U. Bunz, A. D. Schluter, G. Szeimies et al. "Concerning the synthesis of [1.1.1]Propellane" Chem. Ber. volume 122, pp.397-398
- ^ (1998) Organic Syntheses, Coll. Vol. 10, p. 658 (2004); Vol. 75, p.98 Online article.
- ^ Piotr Kaszynski and Josef Michl (1988), [n]Staffanes: a molecular-size "Tinkertoy" construction set for nanotechnology. Preparation of end-functionalized telomers and a polymer of [1.1.1]propellane J. Am. Chem. Soc.; Volume 110 issus 15, pp. 5225 - 5226; doi: 10.1021/ja00223a070
This article is taken from the Japanese Wikipedia 1,1,1-propellane
This article is distributed by cc-by-sa or GFDL license in accordance with the provisions of Wikipedia.
In addition, Tranpedia is simply not responsible for any show is only by translating the writings of foreign licenses that are compatible with CC-BY-SA license information.
0 개의 댓글:
댓글 쓰기