ロンギホレン
| (+) -ロンギホレン | |
|---|---|
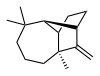 | |
| (1R,2S,7S,9S) - 3,3,7-trimethyl- 8-methylenetricyclo- [5.4.0.02,9]undecane | |
| Identification information | |
| CAS registration number | 475-20-7 |
| ChemSpider | 1406720 |
| It is an address number on a day | J12.093F |
| |
| |
| Characteristic | |
| Chemical formula | C15H24 |
| Molar mass | 204.36 g/mol |
| Density | 0.928 g/cm3 |
| The boiling point | 254 degrees Celsius (706mm Hg) |
| Ratio optical rotation [α]D | + 46 (neat, 22 degrees Celsius) |
| Refractive index (nD) | 1.504 (20 degrees Celsius, D string) |
| I can put a case, the data without the special mention for normal temperature (25 degrees Celsius), the ordinary pressure (100 kPa). | |
ロンギホレン (longifolene) is a kind of the sesquiterpene hydrocarbon expressed in molecular formula C15H24. I have a frame (ロンギホラン frame) of the specific tricyclic nature. The chemical combination tanka with a thing's name in it comes from a thing obtained from the essential oil of the Himalayas pine (considered to be Pinus roxburghii at Pinus longifolia, the present).
Table of contents
Existence
5%–10% degree exists in the essential oil of the Himalayas pine as the chemical combination tanka with a thing's name in it is derived. ロンギホレン is supplied by fractionation of this turpentine. I am contained elsewhere by essential oil such as Mandarin, the cinnamon.
Structure decision
It was isolated in 1920 [1], but the structure decision ran into difficulties because rearrangement reaction was easy to be caused with complicated structure. The structure of the hydrogen chloride addition of ロンギホレン was decided in 1953 by an X-ray crystallographic analysis [2]. Because Wagner Meerwein transposition happens in the case of hydrogen chloride addition, carbon skeleton changes. With regard to it, structure was estimated by chemical reactivity and Raman spectrum of ロンギホレン oneself [3]. As for the estimated structure, a right thing was confirmed by total synthesis [4] that was finally accomplished by Elias Cory in 1961.
Total synthesis
Views about the retrosynthesis analysis are listed in full paper [5] of the total synthesis of Cory in detail. Therefore it is often taken up by a commentary about the retrosynthesis analysis.
The outlines of the total synthesis of Cory are as follows. After synthon is ウィーラント me Shah ketone, and acetal protected the saturated carbonyl group of this selectively, I make an unsaturated carbonyl group ethylidene by Wittig reaction and make ring outside double bond the hydroxy with osmic acid and make a second class hydroxy group tosyl selectively and perform the ring expansion to a 7-membered ring by pinacol rearrangement. Double bond isomerizes it in a conjugated system at the same time when I deprotect acetal here. Three rings of ロンギホレン are completed when I perform the Michael addition in molecules with these two ring-related ketone. After assuming the carbonyl group on the 7-membered ring dithioacetal selectively after having methylated α carbonyl group digit on the 7-membered ring, and having returned the other carbonyl group to alcohol once, I desulfurize it with sodium. I correct the alcohol which I reduced to ketone by the chromate oxidation and methylate it with methyl lithium, and ロンギホレン is provided when I spin-dry it. In addition, it is L-(2S,3S) in becoming it dithioacetal -It is (+) by performing it with 2,3-butanedithiol, and separating a diastereomer by chromatography -I perform the total synthesis of ロンギホレン.
Reactive
I cause Wagner Meerwein transposition by Broensted acid and a Lewis acid easily. By the reaction with halogenation hydrogen, the ninth place is protonated (in IUPAC glossology) and transposes it when carbon of the sixth place is from the seventh place to the eighth place, and the seventh place is halogenated. In addition, multi-stage transposition goes with the non-nucleophilic Broensted acid (for example, sulfate) and Lewis acid (e.g., boron trifluoride - acetic acid) and changes in イソロンギホレン (2,2,7,7-tetramethyl tricyclo[6.2.1.01,6]undec-5-ene). I cause transposition on the severer condition more and change into 1,1-dimethyl-7-isopropyl-1,2,3,4-tetrahydronaphthalene. [6]
Biosynthesis
Because it is sesquiterpene, it is composed life by farnesyl diphosphoric acid. At first a distal portion of alkene which is formed when diphosphoric acid is detached is connected and forms a 11-membered ring and produces an allyl cation by 1,3-hydride movement. Furthermore, two times of cross-linked closures happen and become three ring-related intermediates. When this causes 1,2-alkyl movement on a ring, ロンギホレン is formed.
Use
There are few big uses as it may be said that it is industrial, but is used as the raw materials because the ester which let ketone and イソロンギホレン which oxidized イソロンギホレン add formic acid is used as fragrance having a tree of fragrance. In addition, provided ジロンギホリルボラン is marketed as a reagent as reactive agent for the asymmetric hydroboration by borane and ロンギホレン.
Footnote
- ^ Simonsen J. L. (1920). "LXI.-The constituents of Indian turpentine from Pinus longifolia, Roxb. Part I." J. Chem. Soc. Trans. 117: 570-578. doi: 10.1039/CT9201700570.
- ^ Moffett, R. H.; Rogers, D. (1953). Chem. Ind.: 916.
- ^ a) Naffa, P.; Ourisson, G. (1953). Chem. Ind.: 917.
b) Naffa, P.; Ourisson, G. (1954). Bull. Soc. Chim. It is 21 Fr: 1115. - ^ Corey, E. J.; Ohno, M.; Vatakencherry, P. A.; Mitra, R. B. (1961). "Total Synthesis of d,l-Longifolene." J. Am. Chem. Soc. 83: 1251-1253. doi: 10.1021/ja01466a056.
- ^ Corey, E. J.; Ohno, M.; Mitra, R. B.; Vatakencherry, P. A. (1964). "Total Synthesis of Longifolene." J. Am. Chem. Soc. 86: 478–485. doi: 10.1021/ja01057a039.
- ^ Dev, S. (1981). "Aspects of Longifolene Chemistry. An Example of Another Facet of Natural Products Chemistry." Acc. Chem. Res. 14: 82–88. doi: 10.1021/ar00063a004.
This article is taken from the Japanese Wikipedia ロンギホレン
This article is distributed by cc-by-sa or GFDL license in accordance with the provisions of Wikipedia.
In addition, Tranpedia is simply not responsible for any show is only by translating the writings of foreign licenses that are compatible with CC-BY-SA license information.
0 개의 댓글:
댓글 쓰기